Africa's Ivory Coast deploys new Malaria Vaccine by India's Serum Institute and Oxford University
Wed 17 Jul 2024, 01:13:14
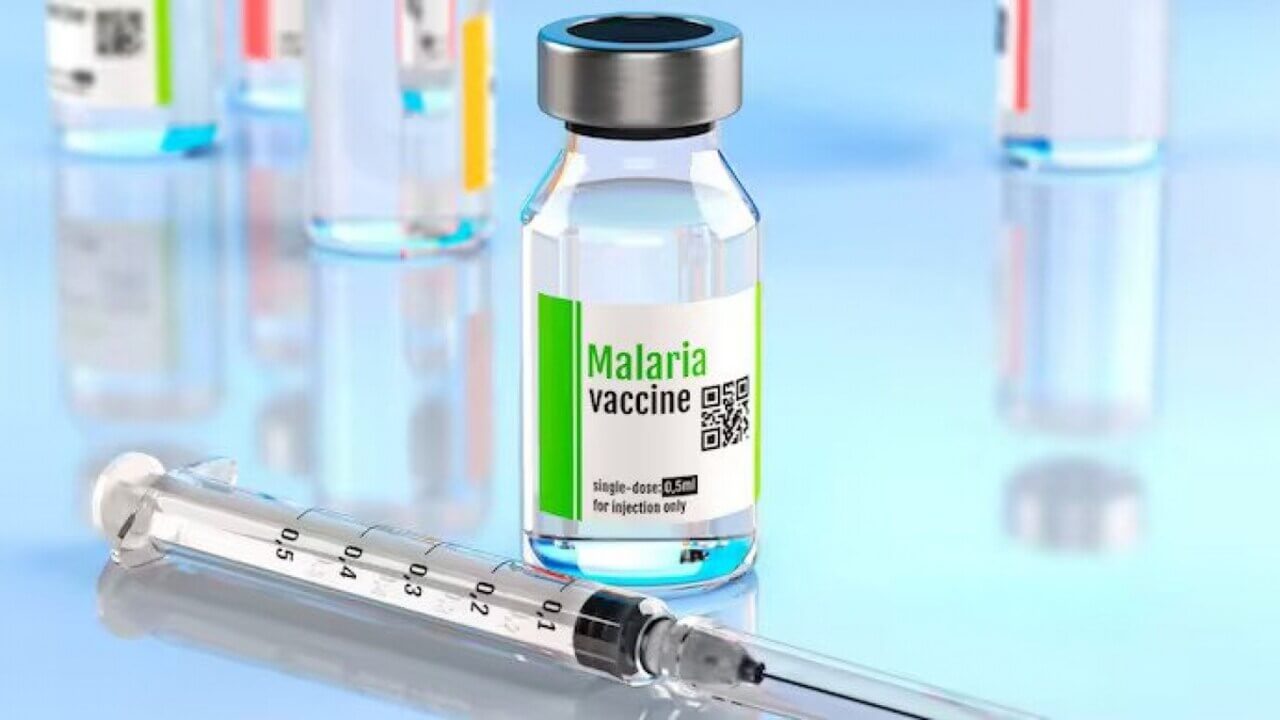
Ivory Coast initiated a routine vaccination programme with the world's second malaria vaccine, R21, developed by the University of Oxford and the Serum Institute of India on Monday. This milestone comes six months after the introduction of the first malaria vaccine, RTS,S, created by British drugmaker GSK, in Cameroon.
The World Health Organisation (WHO) has approved the R21 vaccine, which is now being rolled out in Ivory Coast. This country has received 656,600 doses, aimed at vaccinating 250,000 children aged between 0 and 23 months. The vaccine has also gained approval in Ghana, Nigeria, Burkina Faso, and the Central African Republic. This marks a significant step forward in combating malaria, particularly in Africa, where the disease claims nearly half a million children under the age of five each year.
Support from Gavi Vaccine Alliance
This year, 15 African countries plan to introduce either the RTS,S, or R21 vaccines, with support from the Gavi global vaccine alliance. The rollout of these vaccines is essential as they are expected to complement existing malaria prevention tools, such as bed nets.
Production, Cost Efficiency
The Serum Institute of India, responsible for manufacturing the R21 vaccine, has committed to producing 25 million doses
for the initial rollout and aims to scale up production to 100 million doses annually. The vaccine is priced at less than $4 per dose, aligning with the Institute's goal of delivering affordable vaccines at scale.
for the initial rollout and aims to scale up production to 100 million doses annually. The vaccine is priced at less than $4 per dose, aligning with the Institute's goal of delivering affordable vaccines at scale.
Vaccine Efficacy
In a large trial conducted in February, the R21 vaccine showed promising results, preventing around three-quarters of symptomatic malaria cases in young children within the first year after vaccination. While comparing the two malaria vaccines directly is challenging due to various trial factors, experts agree that both vaccines perform similarly. This assessment is supported by WHO.
Addressing Vaccine Demand
The introduction of a second malaria vaccine is a crucial development in the global fight against the disease. However, experts caution that the demand for these vaccines will likely exceed supply for several years. Therefore, having safe and effective malaria vaccines is critical to meet the high demand and ensure a robust response to malaria.
In summary, the launch of the R21 vaccine in Ivory Coast represents a significant advancement in malaria prevention. It highlights the collaborative efforts of international health organisations, vaccine developers, and manufacturers in tackling one of Africa's most deadly diseases.
No Comments For This Post, Be first to write a Comment.
Most viewed from Health
AIMIM News
Latest Urdu News
Most Viewed
May 26, 2020
Do you think Canada-India relations will improve under New PM Mark Carney?
Latest Videos View All
Like Us
Home
About Us
Advertise With Us
All Polls
Epaper Archives
Privacy Policy
Contact Us
Download Etemaad App
© 2025 Etemaad Daily News, All Rights Reserved.