AstraZeneca, Oxford University resume clinical trials of Covid-19 vaccine
Mon 14 Sep 2020, 10:12:15
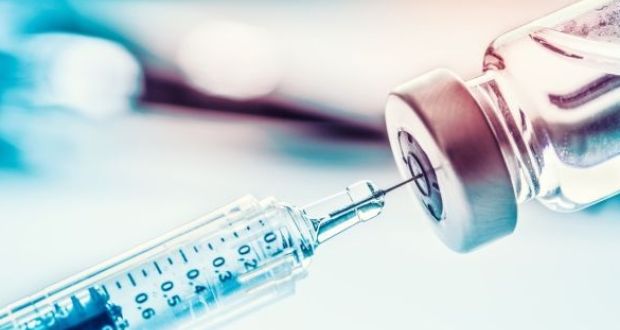
Clinical trials of COVID-19 vaccine being developed by AstraZeneca and Oxford University have resumed in the United Kingdom after a brief safety pause. The trials resumed after the Medicines Health Regulatory Authority confirmed that the trials are safe. AstraZeneca had paused its trials on Tuesday after observing a single event of an unexplained illness that occurred in the UK phase III
trial.
trial.
After Astrazeneca resumed the trial in the United Kingdom, Serum Institute of India has said that they will restart the trial in the country once the apex drug regulator allows. Serum Institute of India partnered with AstraZeneca to manufacture the Oxford COVID-19 vaccine candidate for low-and-middle income countries.
No Comments For This Post, Be first to write a Comment.
Most viewed from Coronavirus Updates
Most viewed from Health
AIMIM News
Asaduddin Owaisi questions PM Modi's China policy
Jan 08, 2025
Owaisi slams UP over police post near Sambhal mosque
Dec 31, 2024
Owaisi hails SC order on Places of Worship Act
Dec 13, 2024
AAP Corporator Tahir Hussain joins AIMIM party
Dec 11, 2024
Latest Urdu News
Most Viewed
May 26, 2020
Which political party will win the Delhi Assembly polls to be held on Feb 5?
Latest Videos View All
Like Us
Home
About Us
Advertise With Us
All Polls
Epaper Archives
Privacy Policy
Contact Us
Download Etemaad App
© 2025 Etemaad Daily News, All Rights Reserved.