COVID-19 treatment: Cipla's remdesivir Cipremi launched in India, to cost ₹4,000
Thu 09 Jul 2020, 16:59:27
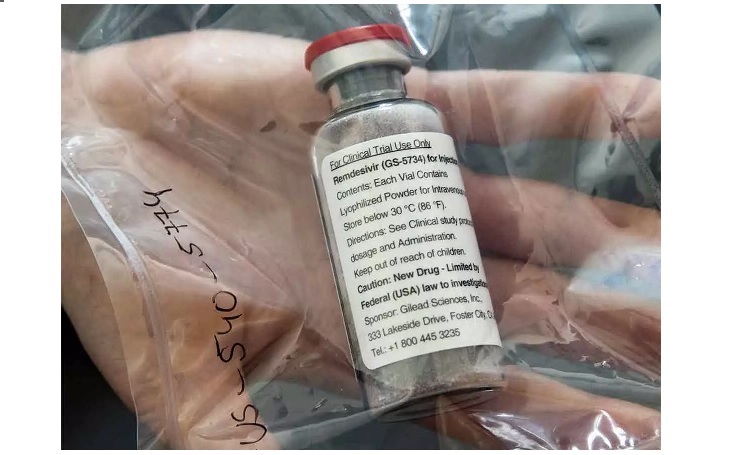
Cipla has launched the generic version of COVID-19 treatment drug remdesivir at a price which is among the lowest globally, according to a PTI report. The pharma major is looking to supply over 80,000 vials within the first month. The drug is priced at ₹4,000 per 100 mg vial, Cipla said in a statement.
Remdesivir is the only drug approved by the United States Food and Drug Administration (USFDA) for Emergency Use Authorisation (EUA) treatment of adult and paediatric patients hospitalised with suspected or laboratory-confirmed COVID-19 infection.
Cipla had earlier said the drug will be priced at less than ₹5,000 per 100 mg vial in line with its overall philosophy of driving access and affordability.
"We are glad to industrially dispatch Cipremi today; among the most minimal evaluated comprehensively, and expect to flexibly more than 80,000 vials inside the main month itself," Cipla Executive VP and CEO (India Business) Nikhil Chopra said in a messaged proclamation to PTI.
To additionally guarantee evenhanded conveyance, the medication will be accessible through government and emergency clinic channels just, the organization included. "Cipla will likewise be giving some measure of the medication as a component of its endeavors to help the network in this period of scarcity," Chopra
said.
said.
Cipremi has been affirmed by the Drug Controller General of India (DCGI) for confined crisis use in the nation as a feature of the quickened endorsement process considering the pressing and neglected clinical need.
Pharmaceutical major Mylan NV on Monday has said that its conventional form of remdesivir will be estimated at ₹4,800 per 100 mg vial. Hyderabad-based medication firm Hetero has said it has fixed the most extreme retail cost of ₹5,400 per vial for the medication.
In May, domestic pharma firms Hetero, Cipla and Jubilant Life Sciences entered into non-exclusive licensing agreements with drug major Gilead Sciences Inc for manufacturing and distribution of remdesivir.
Covaxin, India's first possible vaccine against COVID-19 will soon start its human trial. The process had already been initiated by Nizam's Institute of Medical Sciences (NIMS). Developed by Bharat Biotech, the Covaxin will be tested on over 1,100 people in two phases. The company had a plan to enrol 375 participants to test COVID-19 vaccine candidate this month.
"We will select healthy individuals and draw blood and send the blood samples to designated labs in New Delhi. They will give the green signal. Then the medicine people will examine and the first shot of the vaccine will be given due observation," NIMS director Dr K Manohar told.
No Comments For This Post, Be first to write a Comment.
Most viewed from Coronavirus Updates
Most viewed from Health
AIMIM News
Delhi Assembly polls: Owaisi leads Padyatra in Okhla
Feb 01, 2025
We reject this Waqf Amendment Bill: Asaduddin Owaisi
Jan 30, 2025
Latest Urdu News
Most Viewed
May 26, 2020
Do you think Canada-India relations will improve under New PM Mark Carney?
Latest Videos View All
Like Us
Home
About Us
Advertise With Us
All Polls
Epaper Archives
Privacy Policy
Contact Us
Download Etemaad App
© 2025 Etemaad Daily News, All Rights Reserved.