DCGI clears Covid-19 vaccines for restricted use in emergency
Mon 04 Jan 2021, 10:16:18
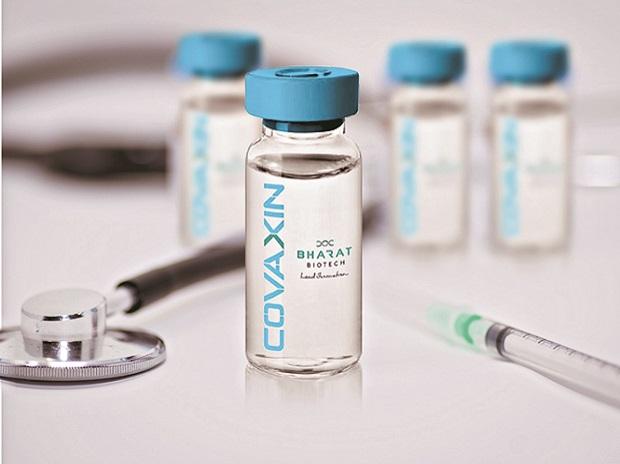
Drugs Controller General of India, DCGI has given approval for restricted use in emergency situation of two Covid19 vaccines- Serum Institute of India's Covishield and Bharat Biotech's Covaxin.
The announcement for granting permission to these two vaccines was made by the Drug Controller General of India VG Somani during a press conference yesterday. COVISHIELD has been developed at the Serum Institute of India's Pune laboratory with a master seed from Oxford University and AstraZeneca.
Two vaccines for coronavirus, Pune-based Serum Institute's Covishield and Bharat Biotech's Covaxin, received emergency approval from the country's drug regulator yesterday. Drug Controller General of India VG Somani said both firms submitted data on their trial runs and both have been granted permission for restricted use. Mr Somani said. DCGI will never approve anything if there is slightest of safety concern. The vaccines are 100 per cent
safe.
safe.
The DCGI said, the Central Drugs Standard Control Organisation (CDSCO) gave its approval followed by the recommendation of the Subject Expert Committee (SEC) that recommended both the vaccines for emergency use.
Prime Minister Narendra Modi has said that the grant of approval by DCGI to the vaccines of Serum Institute of India and Bharat Biotech, accelerates the road to a healthier and COVID-free nation.
Congratulating the nation and the hardworking scientists and innovators of the country, Mr Modi said in a tweet that it is a turning point to strengthen the spirited fight. He said, it would make every Indian proud that the two vaccines that have been given emergency use approval are made in India. The Prime Minister said that this shows the eagerness of our scientific community to fulfill the dream of an Aatmanirbhar Bharat, at the root of which lies care and compassion.
No Comments For This Post, Be first to write a Comment.
Most viewed from Coronavirus Updates
Most viewed from Health
AIMIM News
Asaduddin Owaisi questions PM Modi's China policy
Jan 08, 2025
Owaisi slams UP over police post near Sambhal mosque
Dec 31, 2024
Owaisi hails SC order on Places of Worship Act
Dec 13, 2024
AAP Corporator Tahir Hussain joins AIMIM party
Dec 11, 2024
Latest Urdu News
Most Viewed
May 26, 2020
Which political party will win the Delhi Assembly polls to be held on Feb 5?
Latest Videos View All
Like Us
Home
About Us
Advertise With Us
All Polls
Epaper Archives
Privacy Policy
Contact Us
Download Etemaad App
© 2025 Etemaad Daily News, All Rights Reserved.