India's Lupin to Sell Generic COVID-19 Drug Favipiravir
Wed 05 Aug 2020, 17:12:40
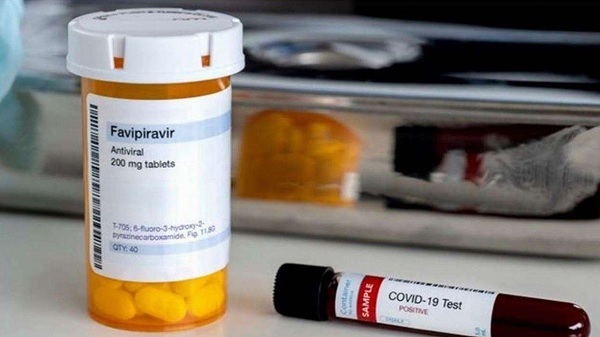
Lupin Ltd on Wednesday became the latest Indian drugmaker to launch a version of the antiviral drug favipiravir to treat COVID-19 in the world’s third most affected country.
A slew of generic drug manufacturers including Cipla Ltd, Sun Pharma and Hetero Labs have been developing favipiravir after India approved it as an as emergency treatment for mild to moderate COVID-19.
Indian hospitals have suffered a shortage of critical COVID-19 drugs such as remdesivir in recent weeks, but the bottlenecks are expected to ease soon as manufacturers ramp up production.
Favipiravir has demonstrated guarantee in clinical preliminaries in India and Russia,
yet faces dubious possibilities in Japan, where it was initially evolved by Fujifilm Holdings, in the wake of baffling clinical examinations.
yet faces dubious possibilities in Japan, where it was initially evolved by Fujifilm Holdings, in the wake of baffling clinical examinations.
Lupin's variant of the medication, called Covihalt, will be valued at 49 rupees (65 pennies) per 200 mg-tablet, it said. Sun Pharma on Tuesday propelled its own rendition, at 35 rupees, so far the least expensive in India.
Shares in Lupin, one of the India’s top generic drug makers, were little changed in midday trading. Coronavirus infections in India topped 1.9 million on Wednesday, after rising by more than 50,000 for seven straight days. The country has the world’s third most affected after the United States and Brazil.
No Comments For This Post, Be first to write a Comment.
Most viewed from Coronavirus Updates
Most viewed from Health
AIMIM News
Delhi Assembly polls: Owaisi leads Padyatra in Okhla
Feb 01, 2025
We reject this Waqf Amendment Bill: Asaduddin Owaisi
Jan 30, 2025
Latest Urdu News
Most Viewed
May 26, 2020
Do you think Canada-India relations will improve under New PM Mark Carney?
Latest Videos View All
Like Us
Home
About Us
Advertise With Us
All Polls
Epaper Archives
Privacy Policy
Contact Us
Download Etemaad App
© 2025 Etemaad Daily News, All Rights Reserved.