Russia announces world's first Covid-19 vaccine, Putin's daughter gets vaccinated
Tue 11 Aug 2020, 16:12:46
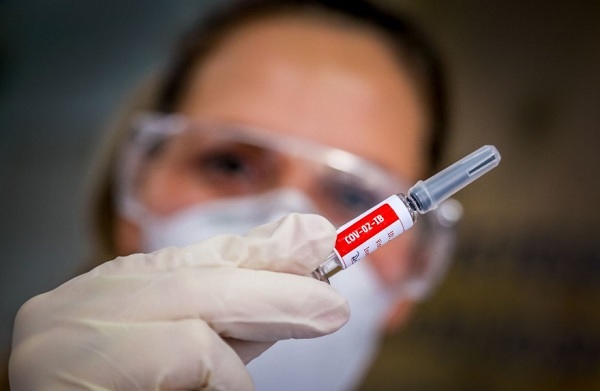
Amid the race to develop a Covid-19 vaccine, Russian President Vladimir Putin launched a coronavirus vaccine, touted as the world's first such vaccine, too. The registration of the vaccine lays ground for mass inoculation even as the final stages of clinical trials to test safety and efficacy continue.
The announcement came in the wake of the novel coronavirus pandemic that has infected more than 20 million people and killed nearly 750,000 worldwide, thus, crippling world economies.
The president has asked Health Minister Mikhail Murashko to keep him informed about the Covid-19 vaccine, while at the same time noting that he knows "it works quite effectively" and "forms a stable immunity", according to Russian news agencies.
"This morning, for the first time in the world, a vaccine against the new coronavirus was registered" in Russia, Putin said during a televised video conference call with government ministers, as per news agency.
Putin further thanked everyone who worked on the vaccine's development and described it as "a very important step for the world". He hopes the country's research body will soon start mass production of coronavirus vaccine.
"I know that it works quite effectively, forms strong immunity, and I repeat, it has passed all the needed checks," said Putin.
He emphasized that the vaccine underwent the necessary tests. He added that one of his two daughters has received a shot of the vaccine and is feeling well, according to Associated Press reports.
However, the vaccine’s registration is conditional and trials will continue while production gets underway, said Murashko.
Moreover, the president has tasked the government with ensuring funding for flu and coronavirus vaccination after vaccines are registered, noting that up to 60% of Russians should be vaccinated against flu.
Russian health ministry had recently said mass vaccinations could begin by October. Murashko said members of “risk groups," such as medical workers, may be offered the vaccine this month.
Russia's first coronavirus vaccine is developed jointly by Gamaleya Research Institute and the Russian Defence Ministry.
Clinical trials of the vaccine began on June 18 and included 38 volunteers. All of the participants developed immunity. The first group was discharged on July 15 and the second group on July 20.
In an earlier report, the final check-up of volunteers testing the coronavirus vaccine showed immunity in all participants, the Russian Defence Ministry said.
Alexander Gintsburg, director of the Gamaleya National Research
Centre, said that vaccine used inanimate particles created on the basis of adenovirus, according to Sputnik News.
Centre, said that vaccine used inanimate particles created on the basis of adenovirus, according to Sputnik News.
He said there are no concerns that the vaccine could potentially cause harm to a person's health. “The particles and objects that can reproduce their own kind are the ones that are considered alive. The particles in question cannot multiply," he added.
Amid the testing and development, Russian health workers treating Covid-19 patients is planned to be offered the chance of volunteering to be vaccinated soon after the vaccine's approval, a source told Reuters last month. Authorities are considering getting doctors and teachers vaccinated against the virus initially as well.
In April, Putin ordered state officials to shorten the time of clinical trials for a variety of drugs, including potential coronavirus vaccines.
However, different industry bodies and pharmaceutical organizations have called Russia's surged enrollment risky. They have scrutinized its broad push to build up an antibody when the current year's end. The turn out of this antibody has mixed worries that it might be putting national glory before science and security.
Dr Anthony Fauci, the top US infectious disease specialist, questioned the fast-track approach last week. “I do hope that the Chinese and the Russians are actually testing a vaccine before they are administering the vaccine to anyone, because claims of having a vaccine ready to distribute before you do testing I think is problematic at best," he said.
The World Health Organization said all vaccine candidates should go through full stages of testing before being rolled out.
More than 100 possible vaccines are being developed around the world to try to stop the pandemic. At least four are in final Phase III human trials, according to WHO data.
Companies including AstraZeneca Plc and Moderna Inc. are still conducting final-stage trials of their vaccines in studies that are expected to soon yield results.
Meanwhile, Philippine President Rodrigo Duterte has accepted Russia’s offer of its coronavirus vaccine, volunteering to take the first shot as a gesture of trust and gratitude. “When the vaccine arrives, I will have myself injected in public. Experiment on me first, that’s fine with me," Duterte said on Monday.
On Tuesday, Russia registered 4,945 new cases of the novel coronavirus, pushing its national case tally to 897,599, the fourth largest in the world.
The official death toll rose to 15,131 after authorities said in their daily coronavirus report that 130 people had died in the previous 24 hours.
No Comments For This Post, Be first to write a Comment.
Most viewed from Coronavirus Updates
Most viewed from Health
AIMIM News
Asaduddin Owaisi questions PM Modi's China policy
Jan 08, 2025
Owaisi slams UP over police post near Sambhal mosque
Dec 31, 2024
Owaisi hails SC order on Places of Worship Act
Dec 13, 2024
AAP Corporator Tahir Hussain joins AIMIM party
Dec 11, 2024
Latest Urdu News
Most Viewed
May 26, 2020
Do you think AAP will perform better in Delhi polls without alliance?
Latest Videos View All
Like Us
Home
About Us
Advertise With Us
All Polls
Epaper Archives
Privacy Policy
Contact Us
Download Etemaad App
© 2025 Etemaad Daily News, All Rights Reserved.