DCGI grants Emergency Use Authorisation to Covid Vaccine Corbevax for children
Tue 22 Feb 2022, 11:31:55
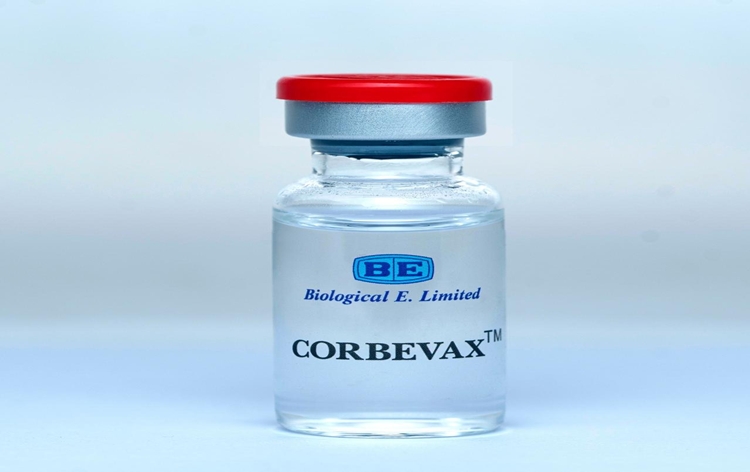
Drugs Controller General of India (DCGI) has granted Emergency Use Authorisation to COVID-19 vaccine Corbevax for children between 12-18 years of age group. The vaccine has been developed by Hyderabad-based pharmaceutical company Biological E Ltd which country's first indigenously developed Receptor Binding Domain protein sub-unit COVID-19 vaccine.
Earlier, the vaccine had received emergency use authorisation for the adult population
in December last year. The Corbevax vaccine is administered through an intramuscular route with two doses scheduled 28 days apart and is stored at 2 to 8 degree Celsius temperature.
in December last year. The Corbevax vaccine is administered through an intramuscular route with two doses scheduled 28 days apart and is stored at 2 to 8 degree Celsius temperature.
Member, Health, NITI Aayog Dr VK Paul said, with the emergency use approval to Corbevax, the country has now two Covid-19 vaccines for children including Covaxin which is already in use for the children aged 15 to 18 years.
No Comments For This Post, Be first to write a Comment.
Most viewed from Health
AIMIM News
Latest Urdu News
Most Viewed
May 26, 2020
Do you think Canada-India relations will improve under New PM Mark Carney?
Latest Videos View All
Like Us
Home
About Us
Advertise With Us
All Polls
Epaper Archives
Privacy Policy
Contact Us
Download Etemaad App
© 2025 Etemaad Daily News, All Rights Reserved.