Here is list of drugs that failed CDCSO's quality test
Fri 27 Sep 2024, 00:08:29

Overall 53 medicines have failed the drug quality test conducted by India's drug regulator. These include paracetamol, calcium, vitamin D3 supplements, anti-diabetes pills and high blood pressure medicines. The Central Drugs Standards Control Organization (CDSCO) has issued a 'Not of Standard Quality' (NSQ) alert for 53 medicines in its monthly drug alert list.
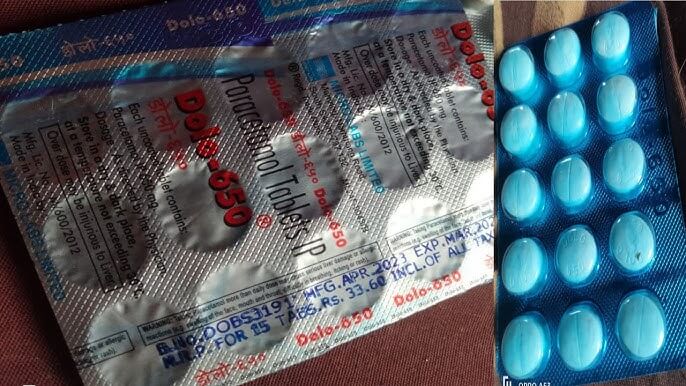
NSQ alerts are generated from random monthly sampling done by state drug officers. The drug regulator's quality tests cover top-selling drugs like Vitamin C, D3 Tablet Shelcal, Vitamin B Complex, Anti Diabetes Pills, Paracetamol Tablet IP 500 MG, anti-diabetic drug Glimepiride and high blood pressure drug Telmisartan.
According to India Today report, these medicines are manufactured by companies like Hetero Drugs, Alkem Laboratories, Hindustan Antibiotics Limited (HAL), Karnataka Antibiotics and Pharmaceuticals Limited, Meg Lifesciences, Pure and Cure Healthcare.
Metronidazole, a drug widely used to treat stomach infections, also failed the quality test. It is manufactured by the government company Hindustan Antibiotics Limited. Similarly, Shelcal, distributed by Torrent Pharmaceuticals, also failed the test. It is manufactured by Uttarakhand-based Pure & Cure Healthcare.
Apart from this, Alkem
Health Science's antibiotics Clavam 625 and Pan D failed the test of a Kolkata lab. The same lab has declared Hyderabad-based Hetero's Cepodem XP 50 dry suspension as substandard. This medicine is for children suffering from serious bacterial infections. Questions have also been raised on the quality of Karnataka Antibiotics and Pharmaceuticals Limited's paracetamol tablets.
Health Science's antibiotics Clavam 625 and Pan D failed the test of a Kolkata lab. The same lab has declared Hyderabad-based Hetero's Cepodem XP 50 dry suspension as substandard. This medicine is for children suffering from serious bacterial infections. Questions have also been raised on the quality of Karnataka Antibiotics and Pharmaceuticals Limited's paracetamol tablets.
The drug regulator has released two lists of medicines that failed the quality test. One list contains 48 medicines. While the other list contains the names of 5 additional medicines. Along with this, the replies of those companies whose medicines failed the test have also been given. In their replies, the companies have refused to take responsibility for these medicines. They have called these medicines fake.
The actual manufacturer (as per the label claim) has informed us that the suspected batch of the product is not manufactured by them and is a counterfeit drug. The product has been claimed to be counterfeit. However, this will be investigated further.
In August, the Central Drugs Standards Control Organization (CDSCO) banned more than 156 fixed-dose drug combinations from the Indian market that were expected to harm people's health. These medicines included fever medicines, painkillers and allergy tablets.
No Comments For This Post, Be first to write a Comment.
Most viewed from Health
AIMIM News
Latest Urdu News
Most Viewed
May 26, 2020
Do you think Canada-India relations will improve under New PM Mark Carney?
Latest Videos View All
Like Us
Home
About Us
Advertise With Us
All Polls
Epaper Archives
Privacy Policy
Contact Us
Download Etemaad App
© 2025 Etemaad Daily News, All Rights Reserved.