Paracetamol and 49 other medicines have been found as substandard by CDSCO
Tue 25 Jun 2024, 00:28:26

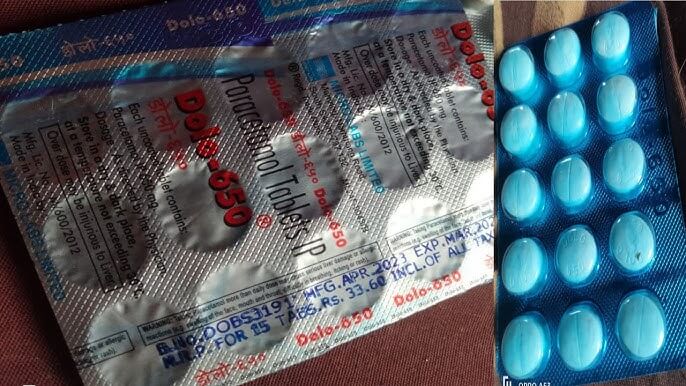
The recent report of the Central Drugs Standard Control Organization (CDSCO) finding 50 medicines, including the commonly used drug Paracetamol, to be substandard has sent shockwaves through the medical community. Paracetamol is a widely prescribed and easily accessible drug that is known for its effectiveness in relieving pain and fever. However, the revelation that it does not meet the quality standards set by the regulatory authorities is indeed alarming.
CDSCO is India's top drug regulator responsible for ensuring the safety, efficacy, and quality of drugs sold in the country. Its findings have revealed that these 50 medicines, including Paracetamol, do not comply with the prescribed standards for various parameters such as dissolution and uniformity. This means that these drugs may not work as intended or may even cause harm to patients.
The 500 mg paracetamol tablets that have been discovered to be subpar are produced by Askon Healthcare, which is situated in Ujjain, Madhya Pradesh. The company states that it has been producing pharmaceutical final dosage forms on its official website.
According to the Mint report, emails and phone calls were made to Askon Healthcare, but
no one responded.
no one responded.
The drug samples were picked from Waghodia (Gujarat), Solan (Himachal Pradesh), Jaipur (Rajasthan), Haridwar (Uttarakhand), Ambala, Indore, Hyderabad, and Andhra Pradesh among other states, according to a Central Drugs Standard Control Organisation (CDSCO) drug alert for May.
According to the HT report, a state drug controller, Manish Kapoor said, “We have received an alert from CODSCO on the samples that failed. From time to time our drug inspectors keep drawing samples of medicines and further action is taken against the erring pharmaceutical companies under the Cosmetic and Drug Act."
“One of every third drugs produced in the country is manufactured in Himachal. The quality of the drugs cannot be compromised,” he also added.
DCGI List
Additional medications on the DCGI list include amlodipine IP tablets and Telmisartan, an antihypertensive medication, Dexamethasone Sodium Phosphate Injection I.P., which is used to treat severe infections and autoimmune diseases, and Clonazepam Tablets, IP, 0.5 mg, which is used to treat neurological complications. Both Central and state laboratories investigated the drug samples.
No Comments For This Post, Be first to write a Comment.
Most viewed from Health
AIMIM News
Latest Urdu News
Most Viewed
May 26, 2020
Do you think Canada-India relations will improve under New PM Mark Carney?
Latest Videos View All
Like Us
Home
About Us
Advertise With Us
All Polls
Epaper Archives
Privacy Policy
Contact Us
Download Etemaad App
© 2025 Etemaad Daily News, All Rights Reserved.