US FDA approves Novo Nordisk's Ozempic to reduce risk of kidney diseases in diabetic patients
Wed 29 Jan 2025, 23:57:01
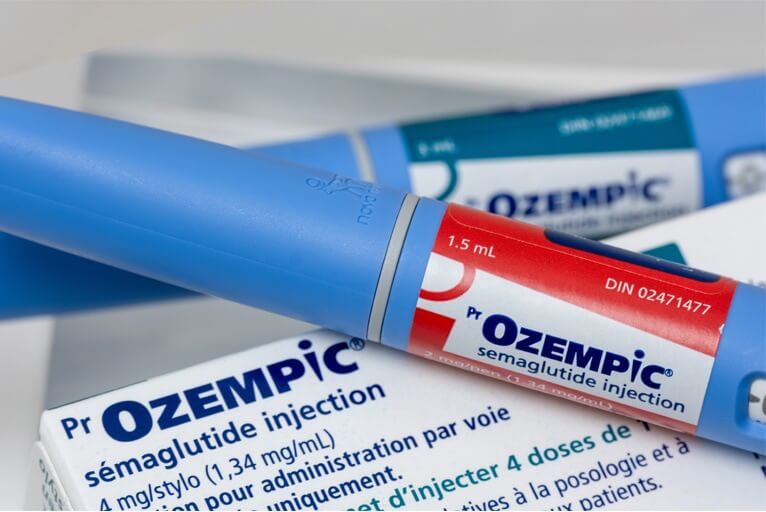
The US Food and Drug Administration (FDA) has approved Novo Nordisk's Ozempic to lower the risk of kidney failure and disease progression. According to a statement made by the Danish pharmaceutical company, approval has also been obtained to minimize the risk of death owing to cardiac difficulties in diabetic patients with chronic kidney disease (CKD).
Ozempic, Novo's blockbuster diabetes medication, belongs to GLP-1 receptor agonists and contains the same active ingredient as its popular obesity treatment, Wegovy. With the FDA's approval, the medicine, chemically known as semaglutide, will become the first GLP-1 treatment option for persons with type 2 diabetes and chronic kidney disease.
“Such an approval adds to the mounting body of evidence showing GLP-1 agents’ utility in indications beyond type 2 diabetes and obesity,” BMO Capital analyst Evan Seigerman said.
The approval was based on findings from late-stage research, which indicated that Ozempic reduced the risk of death from chronic renal disease and significant cardiac events by 24%, the company stated.
Ozempic is also licensed for the prevention of serious cardiovascular events such as heart attacks, strokes, and mortality in persons with diabetes and known heart disease. Last month, the European Medicines Agency approved Novo's request to add risk reduction for renal disease-related events to the Ozempic label.
According to Novo Nordisk, over 40% of persons with type 2 diabetes have chronic renal disease, which affects roughly 37 million adults in the United States. Last year,
the FDA authorized Wegovy to reduce the risk of stroke and heart attack in overweight or obese people who do not have diabetes.
the FDA authorized Wegovy to reduce the risk of stroke and heart attack in overweight or obese people who do not have diabetes.
The business is also investigating the potential benefits of GLP-1 therapy for conditions other than diabetes and weight reduction, such as Alzheimer's disease and non-alcoholic steatohepatitis (NASH).
Novo Nordisk recently published favorable results from the FLOW renal outcomes trial, which found that Ozempic (semaglutide) 1.0 mg lowered the risk of kidney disease progression and kidney and cardiovascular fatalities by 24% in March of last year.
If authorized, Ozempic will be the first GLP-1 therapeutic option for people with type 2 diabetes (T2D) and chronic kidney disease (CKD). GlobalData, a prominent data and analytics business, claimed last year that the medicine could satisfy an unmet need in the kidney disease area because it has a different mode of action than the existing standard of care (SOC) in CKD.
“According to key opinion leaders (KOLs) interviewed by GlobalData, Ozempic could be an excellent option for patients who are diabetic and obese. In addition, KOLs believe that Ozempic has the potential to be a treatment option for non-diabetic CKD patients whose kidney problems are due to their obesity as opposed to diabetes,” Kajal Jaddoo, Senior Pharma Analyst at GlobalData, said.
She further added that Ozempic, a GLP-1 receptor agonist, is used in conjunction with diet and exercise to enhance glycemic control in persons with type 2 diabetes. It is designed as an injectable solution for subcutaneous delivery.
No Comments For This Post, Be first to write a Comment.
Most viewed from Health
AIMIM News
Latest Urdu News
Most Viewed
May 26, 2020
Do you think Canada-India relations will improve under New PM Mark Carney?
Latest Videos View All
Like Us
Home
About Us
Advertise With Us
All Polls
Epaper Archives
Privacy Policy
Contact Us
Download Etemaad App
© 2025 Etemaad Daily News, All Rights Reserved.