US 's FDA approved first respiratory syncytial virus vaccine for use in women during pregnancy to protect baby
Wed 23 Aug 2023, 10:18:49
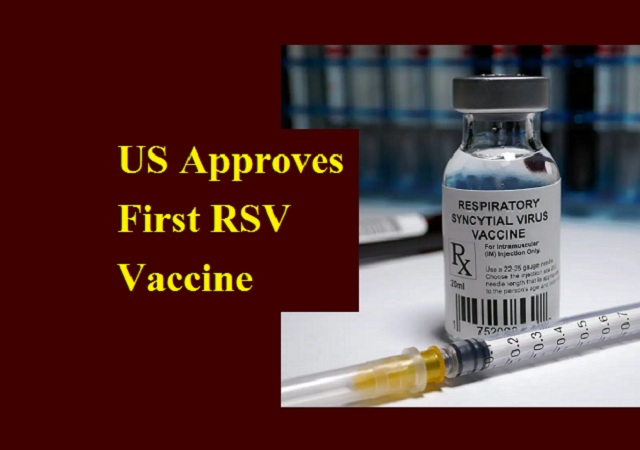
The United States of America's Food and Drug Administration (FDA) has approved the first respiratory syncytial virus (RSV) vaccine for use in women during pregnancy to protect the baby. This vaccine has been developed by Pfizer and the approval allows the vaccine to be given to women 32 to 36 weeks into pregnancy for prevention of lower respiratory tract infection and severe
disease in infants until they are six months old.
disease in infants until they are six months old.
The vaccine developer has said that maternal vaccination could prevent a large number of hospitalizations due to RSV. The syncytial virus is said to be a common cause of illness in children, while infants are those at the highest risk for severe disease.
No Comments For This Post, Be first to write a Comment.
Most viewed from Health
AIMIM News
Latest Urdu News
Most Viewed
May 26, 2020
Should there be an India-Pakistan cricket match or not?
Latest Videos View All
Like Us
Home
About Us
Advertise With Us
All Polls
Epaper Archives
Privacy Policy
Contact Us
Download Etemaad App
© 2026 Etemaad Daily News, All Rights Reserved.






















.jpg)
.jpg)
.jpg)


