Zika vaccine comes closer to reality
Thu 11 May 2017, 21:07:08
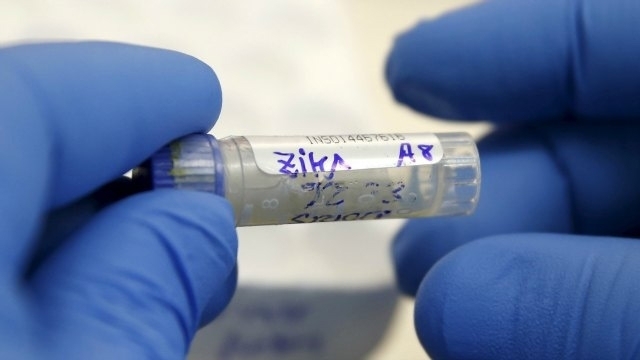
Washington D.C. [USA], May 11 : A new study has paved the way for a promising vaccine for the Zika virus.
Preclinical results of research by City College of New York scientists and TechnoVax, Inc. in animal models demonstrated favourable outcomes in developing a vaccine against the mosquito-borne Zika virus.
The results were announced by Tarrytown, New York-based TechnoVax, a biotechnology developer of novel vaccines whose proprietary virus-like particle (VLP) is the centre of the research.
The VLP vaccine formulations tested in animals not only were highly effective in eliciting
protective antibodies with neutralizing activity equivalent to or higher than the activity present in the serum of a patient who recovered from Zika infection but also were well tolerated and safe.
protective antibodies with neutralizing activity equivalent to or higher than the activity present in the serum of a patient who recovered from Zika infection but also were well tolerated and safe.
Jose M. Galarza, TechnoVax CEO, noted that the ZIKA VLP vaccine offers an effective and safe strategy to create a prophylactic vaccine that protect against Zika infection as well as its serious effects such as microcephaly.
The ultimate goal of the collaboration is to expand the translational research with TechnoVax by completing the Zika vaccine development and initiate new vaccine projects directed to additional virus pathogens.
No Comments For This Post, Be first to write a Comment.
Most viewed from Health
AIMIM News
Latest Urdu News
Most Viewed
May 26, 2020
Do you think Canada-India relations will improve under New PM Mark Carney?
Latest Videos View All
Like Us
Home
About Us
Advertise With Us
All Polls
Epaper Archives
Privacy Policy
Contact Us
Download Etemaad App
© 2025 Etemaad Daily News, All Rights Reserved.