Bangladesh Approves Late-stage Trial of China's Sinovac COVID-19 Vaccine
Mon 20 Jul 2020, 15:37:53
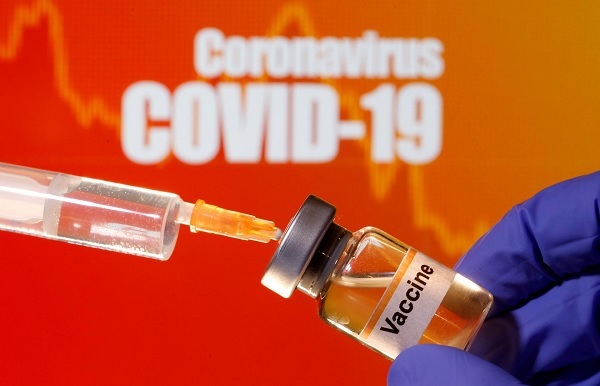
Bangladesh has approved the third-phase trial of a potential COVID-19 vaccine developed by China's Sinovac Biotech Ltd, officials said on Monday, as infections continue to rise in the densely-populated South Asian country.
Sinovac has been searching for volunteers outside of China as the quantity of coronavirus cases there has dwindled, said an individual from Bangladesh's national specialized warning advisory group to handle COVID-19.
The International Center for Diarrhoeal Disease Research, Bangladesh (ICDDR,B), will lead the preliminary that could start one month from now.
“We have given ethical permission for the trial after reviewing the research protocol,” Mahmood Uz Jahan, director
of the Bangladesh Medical Research Council (BMRC), told Reuters.
of the Bangladesh Medical Research Council (BMRC), told Reuters.
"The protocol given to BMRC by ICDDR,B will be applied to 4,200 volunteers. Half of them will get vaccinated."
The trial would be conducted in seven COVID-19 hospitals in Dhaka, Bangladesh's capital, an ICDDR,B official said on the condition of anonymity.
The country had 204,525 confirmed coronavirus cases as of Sunday, with 2,618 deaths.
Sinovac said this month it was starting Phase III trials of its potential coronavirus vaccine in Brazil.
A senior Bangladesh health ministry official said the country hoped to get priority in securing the vaccine should it prove effective in the trials.
No Comments For This Post, Be first to write a Comment.
Most viewed from International
Most viewed from World
AIMIM News
Latest Urdu News
Most Viewed
May 26, 2020
Do you think Canada-India relations will improve under New PM Mark Carney?
Latest Videos View All
Like Us
Home
About Us
Advertise With Us
All Polls
Epaper Archives
Privacy Policy
Contact Us
Download Etemaad App
© 2025 Etemaad Daily News, All Rights Reserved.



.jpg)





.jpg)
.jpg)







.jpg)
.jpg)
.jpg)
.jpg)
.jpg)

















