British woman is first in the world to undergo gene therapy for most common form of blindness
Tue 19 Feb 2019, 11:37:51
After years of people fleeing Syria and its civil war, there are now long queues to enter the country each day. Jordan opened its Jaber border crossing last October after Syrian government troops defeated rebels who had controlled the other side.
Now several thousand people pass through each day. They include small-scale merchants reviving cross-border trade and returning Syrian refugees who hope to rebuild their lives.
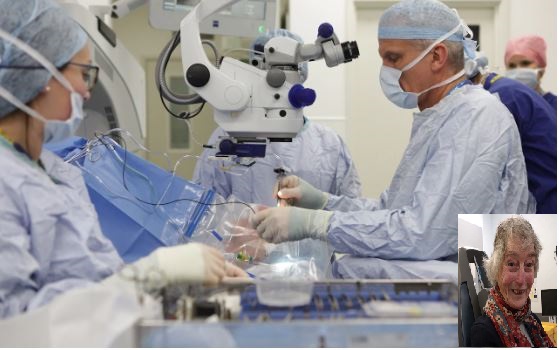
Middle East Correspondent Yolande Knell spent a day at the crossing. A British woman has become the first person in the world to undergo gene therapy for the most common cause of sight loss.
Surgeons at the John Radcliffe Hospital in Oxford inserted a synthetic gene into the left eye of Janet Osborne, 80, who suffers from age-related macular degeneration (AMD).
Around 600,000 people in the UK are affected by AMD, which affects the central part of a patient’s vision with gaps or ‘smudges’, making everyday activities like reading and recognising faces difficult.
Mrs Osborne of Oxford, has AMD in both eyes but it is more advanced in the left, leaving her struggling to prepare food, sew and read.
She says her motivation for taking part in the trial was the possibility of helping others with AMD: “I wasn’t thinking of me. I was thinking of other people.
“For me, I hope my sight doesn’t get any worse. That would be fantastic. It means I wouldn’t be such a nuisance to my family.”
The operation involves detaching the retina and injecting a solution containing a harmless virus underneath.
The virus contains a modified DNA sequence, which infects cells, called the retinal pigment epithelium (RPE), and corrects a genetic defect that causes AMD.
“AMD is the number one cause of untreatable blindness in the developed world,” said
Prof MacLaren.
Prof MacLaren.
“A genetic treatment administered early on to preserve the vision in patients who would otherwise lose their sight would be a tremendous breakthrough and certainly something I hope to see in the near future.”
The operation was part of the FOCUS trial, sponsored by Gyroscope Therapeutics, a UK biotech company developing gene therapy products for ocular diseases such as dry AMD.
AMD occurs because of an over-production of proteins produced by the immune system to fight bacteria, which start to attack cells in the retina in a similar way to how they would attack disease.
Prof MacLaren said : “The idea of this gene therapy is to ‘deactivate’ the (immune system), but at a very specific point at the back of the eye, so the patient would otherwise be unaffected by it, and we hope that in future it will slow down the progression of macular degeneration.”
Sir Peter Lachman, the scientist from the University of Cambridge who led the pioneering work on the complement system leading to the creation of Gyroscope Therapeutics, said: “We have a better understanding now on the relationship between the complement system and the AMD disease which lead us to the discovery that restoring the balance of a hyperactive complement system could be a potential therapeutic approach in dry AMD”.
The aim of the therapy is not to restore sight but to halt the progress of the condition and preserve what vision patients have remaining.
If successful, it is hoped that gene therapy can be used in the future on patients with early AMD and so halt the disease before their vision has started to deteriorate.
Prof MacLaren added: “This is a rapidly evolving field. Given that we understand a lot more now about the manufacture of the treatment, and the effects of the virus when doing gene therapy at the back of the eye, as well as all the other gene therapy programmes being developed at the moment, I would hope that we’ll see a treatment for people with dry AMD within the next few years."
The trial was carried out with the support of the NIHR Oxford Biomedical Research Centre.
No Comments For This Post, Be first to write a Comment.
Most viewed from International
Most viewed from World
AIMIM News
Latest Urdu News
Most Viewed
May 26, 2020
Do you think Canada-India relations will improve under New PM Mark Carney?
Latest Videos View All
Like Us
Home
About Us
Advertise With Us
All Polls
Epaper Archives
Privacy Policy
Contact Us
Download Etemaad App
© 2025 Etemaad Daily News, All Rights Reserved.

.jpg)
.jpg)
.jpg)








.jpg)
.jpg)
.jpg)
.jpg)
.png)
.jpg)

.jpg)
.jpg)
.jpg)
.jpg)
.jpg)


















