China-made covid vaccine could be available earlier than expected
Thu 23 Jul 2020, 16:19:17
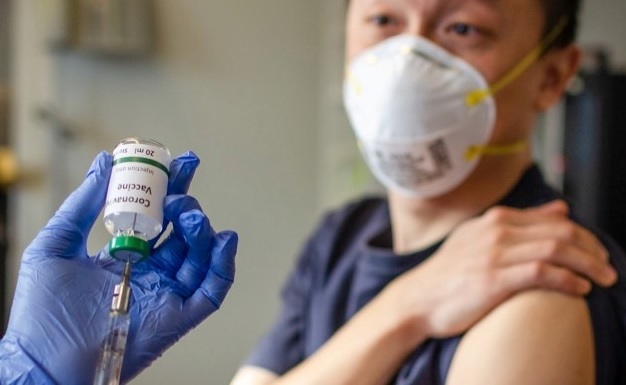
A COVID-19 vaccine candidate developed by Chinese state-owned pharmaceutical company Sinopharm could be ready ahead of previous expectations. It could be available for public use by the end of this year, Reuters reported, citing state media. Sinopharm had said in June the vaccine shot may not be ready until at least 2021 as a lack of new infections in China made it difficult to find people to test it on.
In any case, from that point forward China has since discovered elective preliminary destinations abroad in the worldwide race to deliver an immunization to battle a pandemic that has murdered more than 6 lakh individuals all inclusive.
Sinopharm Chairman Liu Jingzhen told state supporter CCTV the organization hopes to complete late-stage human testing inside around a quarter of a year, Reuters
announced. Not long ago, Sinopharm has started Phase III clinical preliminaries of a COVID-19 antibody in Abu Dhabi utilizing something like 15,000 volunteers.
announced. Not long ago, Sinopharm has started Phase III clinical preliminaries of a COVID-19 antibody in Abu Dhabi utilizing something like 15,000 volunteers.
The experimental vaccine passed Phases I and II of clinical trials with 100% of volunteers generating antibodies after two doses in 28 days, an Abu Dhabi government statement said.
Another potential vaccine, developed by Chinese firm Sinovac Biotech is also into the final-stage trial in Brazil.
Another experimental vaccine, developed by Oxford University and pharmaceutical firm AstraZeneca, is also in Phase 3 testing in Brazil.
Studies published on Monday in British medical journal The Lancet found the Oxford vaccine to be safe and produced an immune response in early stage trials.
No Comments For This Post, Be first to write a Comment.
Most viewed from International
Most viewed from World
AIMIM News
Latest Urdu News
Most Viewed
May 26, 2020
Do you think Canada-India relations will improve under New PM Mark Carney?
Latest Videos View All
Like Us
Home
About Us
Advertise With Us
All Polls
Epaper Archives
Privacy Policy
Contact Us
Download Etemaad App
© 2025 Etemaad Daily News, All Rights Reserved.



.jpg)





.jpg)
.jpg)







.jpg)
.jpg)
.jpg)
.jpg)
.jpg)

















