New vaccine developed for Chikungunya by French biotech Valneva
Tue 13 Jun 2023, 18:21:50
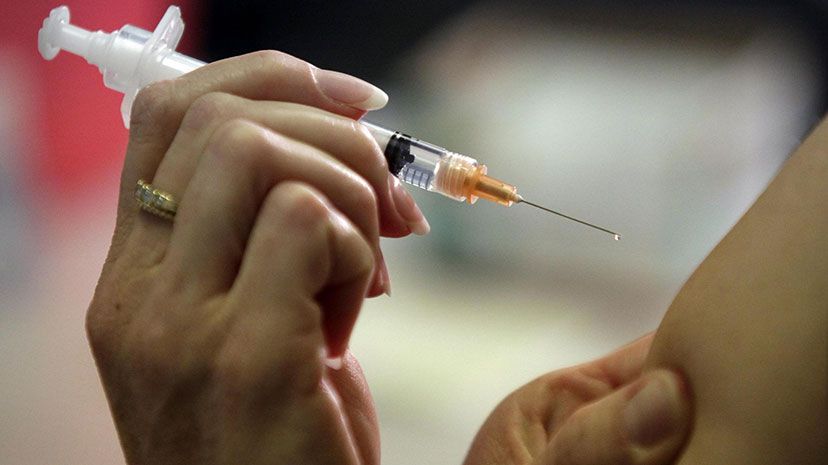
New York: New single dose chikungunya vaccine developed by French biotech Valneva is safe and produces an immune response, according to a phase 3 randomised controlled trial published in The Lancet.
After a single vaccination, VLA1553 induced antibody levels at a level that is
considered to protect against disease among 99 percent (263/266) of participants. There was no difference in immune response according to age.
considered to protect against disease among 99 percent (263/266) of participants. There was no difference in immune response according to age.
While antibody levels declined 28 days after vaccination, seroprotection persisted in more than 96 per cent (233/242) participants after six months.
No Comments For This Post, Be first to write a Comment.
Most viewed from International
Most viewed from World
AIMIM News
Latest Urdu News
Most Viewed
May 26, 2020
Is it right to exclude Bangladesh from the T20 World Cup?
Latest Videos View All
Like Us
Home
About Us
Advertise With Us
All Polls
Epaper Archives
Privacy Policy
Contact Us
Download Etemaad App
© 2026 Etemaad Daily News, All Rights Reserved.




































.jpg)
.jpg)
.jpg)


