UK backs COVID-19 Vaccine Trials that will Inject Healthy Volunteers with Virus
Tue 20 Oct 2020, 15:15:31
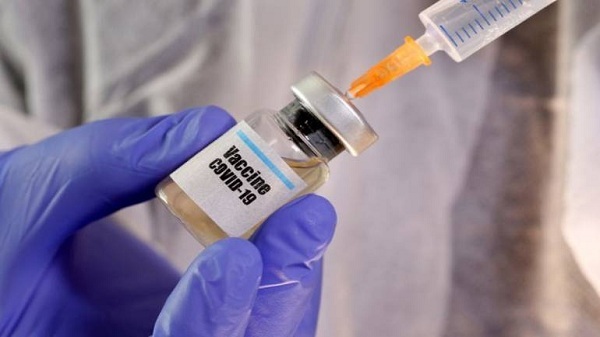
United Kingdom on Tuesday said that it will support country's "human challenge" trials, where young and healthy volunteers are deliberately infected with COVID-19, to accelerate the development of Covid-19 vaccines .
The government said it would invest 33.6 million pounds ($43.5 million) in the studies in partnership with Imperial College London, laboratory and trial services company hVIVO and the Royal Free London NHS Foundation Trust, reported Reuters.
If approved by regulators and an ethics committee, the studies would start in January with results expected by May 2021, the government said.
Britain's hVIVO, a unit of pharmaceutical services company Open Orphan, said on Friday it was carrying out preliminary work for the trials.
Using controlled doses of virus, the aim of the research team will initially be to discover the smallest amount of virus it takes to cause COVID-19 infection in small groups of healthy young people, aged between 18 and 30, who are at the lowest risk of harm, the government said.
Up to 90 volunteers could be involved at the early stage, it said.
Imperial College's Chris Chiu, lead researcher on the human challenge study, said the trials could increase understanding of COVID-19 in unique ways and accelerate development of the many potential new treatments and vaccines.
"Our number one priority is the safety of the volunteers," he said. "My team has been safely running human challenge studies with other respiratory viruses for over 10 years. No study is completely risk free, but the Human Challenge Programme partners will be working hard to ensure we make the risks
as low as we possibly can."
as low as we possibly can."
The researchers hope that the work will ultimately help to reduce the spread of the coronavirus, mitigate its impact and reduce deaths from COVID-19.
The first stage of the project will explore the feasibility of exposing healthy volunteers to the SARS-CoV-2 coronavirus. The study would recruit volunteers between the ages of 18 and 30 with no previous history or symptoms of COVID-19, no underlying health conditions and no known adverse risk factors for COVID-19, such as heart disease, diabetes or obesity.
In this initial phase, the aim will be to discover the smallest amount of virus it takes to cause a person to develop COVID-19. This is known as a virus characterisation study.
When this first stage is finished, clinical analysts mean to utilize this human test model to concentrate how immunizations work in the body to stop or forestall COVID-19, to take a gander at possible medicines and study the resistant reaction.
Human test concentrates additionally make it workable for researchers to look at the viability of immunization applicants by testing them one next to the other to set up which is more viable. At this beginning phase no particular immunization contender for the human test preliminaries have been affirmed, educated Imperial College regarding London through a public statement.
Meanwhile, another 18,804 people in Britain tested positive for COVID-19, bringing the total number of coronavirus cases in the country to 741,212, according to official figures released Monday.
The coronavirus-related deaths in Britain rose by 80 to 43,726, the data showed.
No Comments For This Post, Be first to write a Comment.
Most viewed from International
Most viewed from World
AIMIM News
Latest Urdu News
Most Viewed
May 26, 2020
Do you think Canada-India relations will improve under New PM Mark Carney?
Latest Videos View All
Like Us
Home
About Us
Advertise With Us
All Polls
Epaper Archives
Privacy Policy
Contact Us
Download Etemaad App
© 2025 Etemaad Daily News, All Rights Reserved.



.jpg)





.jpg)
.jpg)







.jpg)
.jpg)
.jpg)
.jpg)
.jpg)

















