DCGI grants approval to Bharat Biotech's nasal Covid booster dose for trials
Wed 05 Jan 2022, 17:59:30
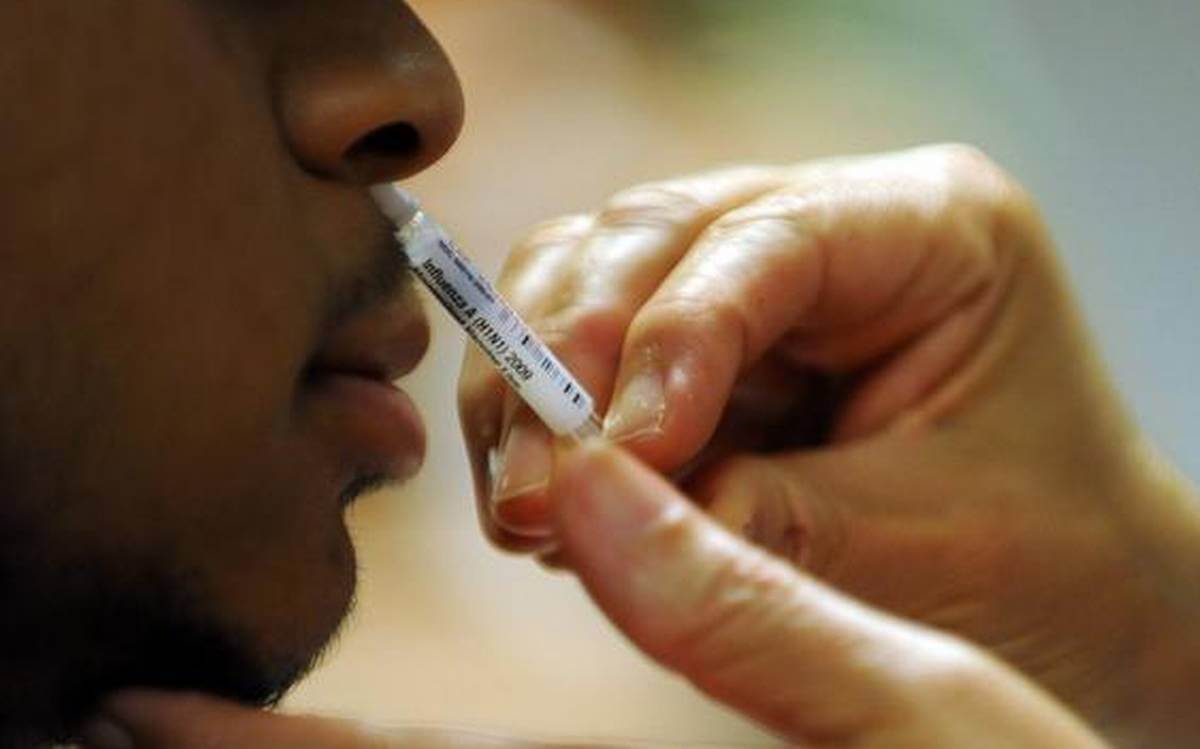
DCGI's Subject Expert Committee (SEC) has granted 'in principle' approval to Bharat Biotech for the conduct of 'Phase III superiority study and Phase III booster dose study' for its intranasal COVID vaccine and has asked it to submit protocols for approval, according to news agency ANI.
According to reports, the Hyderabad-based biotechnology company had earlier proposed that its nasal vaccine can be used as a booster dose for those who have already been inoculated with Covaxin or Covishield vaccines against coronavirus.
Bharat
Biotech aims to conduct clinical trials on 5,000 subjects (50 per cent vaccinated with Covishield and 50 per cent vaccinated with Covaxin).
Biotech aims to conduct clinical trials on 5,000 subjects (50 per cent vaccinated with Covishield and 50 per cent vaccinated with Covaxin).
The interval between the second dose and booster dose will be six months, sources have told. According to the sources, India is expected to get an Intranasal booster vaccine in March, after timely conduction of trials.
The government had recently approved Serum Institute of India's vaccine Covovax, Biological E's jab Corbevax and anti-Covid pill Molnupiravir expanding India's basket of COVID-19 vaccines.
No Comments For This Post, Be first to write a Comment.
Most viewed from National
Most viewed from World
AIMIM News
Latest Urdu News
Most Viewed
May 26, 2020
Which Cricket team will win the IPL 2025 trophy?
Latest Videos View All
Like Us
Home
About Us
Advertise With Us
All Polls
Epaper Archives
Privacy Policy
Contact Us
Download Etemaad App
© 2025 Etemaad Daily News, All Rights Reserved.

.jpg)
.jpg)
.jpg)
.jpg)








.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)






















