Govt informs clinical trials of Covaxin for 2-18 age group to begin in next 10-12 days
Tue 18 May 2021, 22:00:51
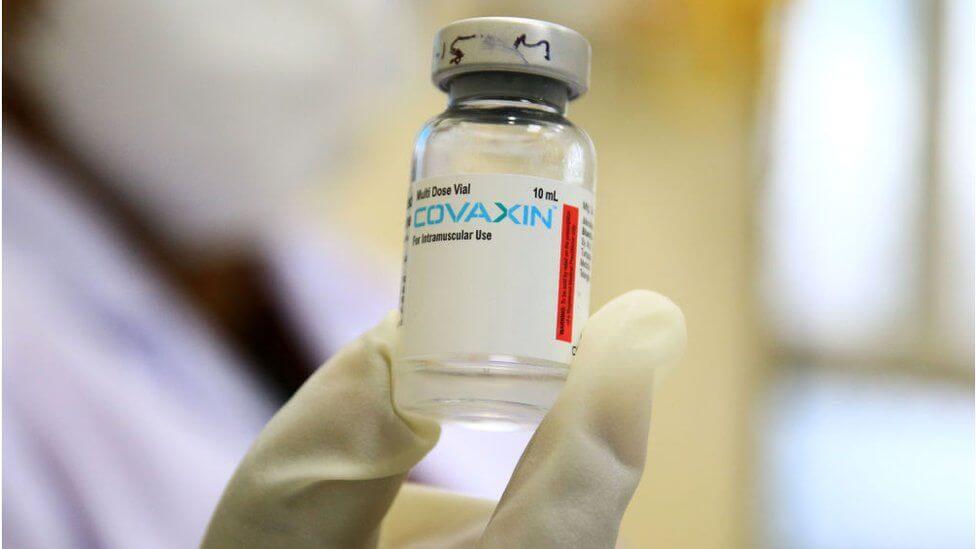
The clinical trials of Bharat Biotech's Covaxin in the age group of 2-18 years will begin in next 10-12 days, the government said Tuesday. The development comes days after India's apex drugs regulator granted permission for conducting the phase II/III clinical trials on children.
"COVAXIN has been approved by the Drugs Controller General of India (DCGI), for Phase II/III clinical trials in the age group of 2 to 18 years.
I have been told that trials will begin in the next 10-12 days," Dr. VK Paul, Member-Health, Niti Aayog said at a press conference today.
I have been told that trials will begin in the next 10-12 days," Dr. VK Paul, Member-Health, Niti Aayog said at a press conference today.
Hyderabad based Bharat Biotech had proposed to carry out the clinical trial in the age group of 2 to 18 years. In the trial, the vaccine will be given by intramuscular route in two doses at day 0 and day 28.
The trial will be conducted on 525 healthy volunteers.
No Comments For This Post, Be first to write a Comment.
Most viewed from National
Most viewed from World
AIMIM News
Asaduddin Owaisi questions PM Modi's China policy
Jan 08, 2025
Owaisi slams UP over police post near Sambhal mosque
Dec 31, 2024
Owaisi hails SC order on Places of Worship Act
Dec 13, 2024
AAP Corporator Tahir Hussain joins AIMIM party
Dec 11, 2024
Latest Urdu News
Most Viewed
May 26, 2020
Which political party will win the Delhi Assembly polls to be held on Feb 5?
Latest Videos View All
Like Us
Home
About Us
Advertise With Us
All Polls
Epaper Archives
Privacy Policy
Contact Us
Download Etemaad App
© 2025 Etemaad Daily News, All Rights Reserved.