Russian Sputnik V to be Tested on 100 Indian Volunteers: COVID Vaccine
Fri 23 Oct 2020, 17:20:54
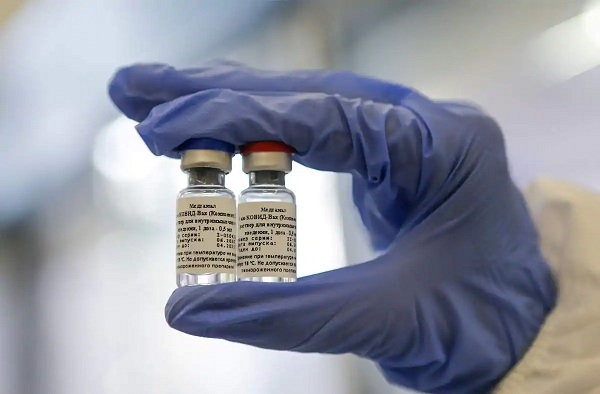
The Russian Sputnik V vaccine against the novel coronavirus will be tested in India on 100 volunteers, the Indian Central Drugs Standard Control Organisation's Drug Controller General (DCGI) told Sputnik.
DCGI has granted permission to pharmaceutical giant Dr Reddy's Laboratories for conducting tests. However, the date and time of the test will be determined by the company.
Sputnik quoted the organisation as saying that the vaccine will be tested in the second phase of its clinical trials before moving on to phase 3.
Last week, the expert committee of DCGI had recommended granting permission to Dr Reddy's Laboratories for conducting phase 2 clinical trials of Russian COVID-19 vaccine candidate, Sputnik V, in India.
According to a government official, Dr Reddy's Lab has stated that in phase 2 clinical trial--"would include 100 subjects and for phase 3, it would take 1400 subjects."
"Once the pharma company would submit the safety and immunogenicity data of phase 2, it would be analysed by the expert panel and then they can proceed for the phase 3 trial," the official added.
On October 13, news agency had reported that Dr Reddy's Laboratories re-applied fresh protocol to DCGI in order to seek its approval for conducting phase 2 and 3 clinical trials of the Russian COVID-19 vaccine.
It may be noted that on October 5, the Subject Expert Committee (SEC) had done a thorough evaluation of the previous application submitted by Dr Reddy's lab. Thereafter, the SEC had directed the pharma company to apply with a revised protocol along with more information.
The Indian drugmaker has joined hands with the Russian Direct Investment Fund (RDIF) to conduct clinical
trials of the Sputnik V vaccine as well as its distribution.As per the RDIF, it will supply 100 million doses of its potential COVID-19 vaccine to India drug company Dr Reddy's Lab.
trials of the Sputnik V vaccine as well as its distribution.As per the RDIF, it will supply 100 million doses of its potential COVID-19 vaccine to India drug company Dr Reddy's Lab.
Last month, Kirill Dmitriev, CEO, RDIF informed that Russia is in close dialogue with the Indian government and drug manufacturers of India regarding localisation of production of its Sputnik V vaccine in India.
Likewise, a renowned clinical diary The Lancet had distributed the consequences of clinical preliminaries of Phase I-II of the Russian immunization showing its security and viability.
On August 11, the Sputnik V antibody applicant grew mutually by RDIF and the Gamaleya National Research Center of Epidemiology and Microbiology was enrolled by the Ministry of Health of Russia and turned into the world's originally enlisted immunization against COVID-19.
As indicated by Russian specialists, Sputnik V is a human adenoviral vector antibody that battles against Covid illness.
India's DCGI's Subject Expert Committee (SEC) recommended granting of permission to conduct Phase III clinical trials for Bharat Biotech's Covid-19 vaccine Covaxin.
The panel added that the recommendation is after assessing data from Phase I & II as well as animal challenge study.
In July, Bharat Biotech had received DCGI nod to conduct phase 1 and 2 clinical trials of an indigenous vaccine for COVID-19.
Meanwhile, India on Friday registered as many as 54,366 fresh Covid-19 cases, taking the overall tally to 77,61,312, as per the Health Ministry's data. The country also recorded 690 more fatalities in a span of 24 hours, taking the virus death toll to 1,17,306.
No Comments For This Post, Be first to write a Comment.
Most viewed from National
Most viewed from World
AIMIM News
Delhi Assembly polls: Owaisi leads Padyatra in Okhla
Feb 01, 2025
We reject this Waqf Amendment Bill: Asaduddin Owaisi
Jan 30, 2025
Latest Urdu News
Most Viewed
May 26, 2020
Which team will win the ICC Men's Champions Trophy 2025 held in Pakistan/Dubai?
Latest Videos View All
Like Us
Home
About Us
Advertise With Us
All Polls
Epaper Archives
Privacy Policy
Contact Us
Download Etemaad App
© 2025 Etemaad Daily News, All Rights Reserved.

.jpg)
.jpg)

.jpg)












.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)

















